Unveiling the mysteries of gas behavior, the combined gas law worksheet 1 answer key provides a comprehensive guide to mastering this fundamental concept. Through a captivating journey of exploration, we delve into the formula, unravel its applications, and conquer worksheet problems with ease, unlocking the secrets of gas transformations in the process.
The combined gas law, a cornerstone of chemistry, allows us to predict gas behavior under varying conditions, empowering us to analyze and understand gas reactions in diverse settings. This worksheet serves as a practical tool, equipping you with the knowledge and skills to navigate the complexities of gas laws with confidence.
Combined Gas Law: Combined Gas Law Worksheet 1 Answer Key
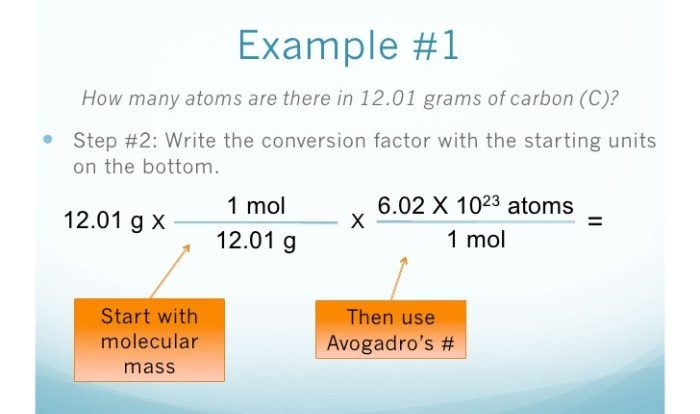
The combined gas law is a mathematical equation that relates the pressure, volume, and temperature of a gas. It is a combination of Boyle’s law, Charles’s law, and Gay-Lussac’s law.
Combined Gas Law Formula
The combined gas law formula is:
(P1V 1)/T 1= (P 2V 2)/T 2
where:
- P is the pressure of the gas in pascals (Pa)
- V is the volume of the gas in cubic meters (m 3)
- T is the temperature of the gas in kelvins (K)
- 1 and 2 refer to the initial and final states of the gas, respectively
Example, Combined gas law worksheet 1 answer key
A gas has a pressure of 100 kPa, a volume of 2 m 3, and a temperature of 298 K. If the pressure is increased to 200 kPa and the volume is decreased to 1 m 3, what is the new temperature of the gas?
Using the combined gas law formula:
(P1V 1)/T 1= (P 2V 2)/T 2
and substituting the given values:
(100 kPa x 2 m3)/298 K = (200 kPa x 1 m 3)/T 2
Solving for T 2:
T2= (200 kPa x 1 m 3x 298 K)/(100 kPa x 2 m 3) = 298 K
Therefore, the new temperature of the gas is 298 K.
Key Questions Answered
What is the combined gas law?
The combined gas law is a mathematical equation that describes the relationship between pressure, volume, temperature, and the number of moles of a gas.
How can I use the combined gas law?
The combined gas law can be used to solve a variety of problems involving gas behavior, such as predicting the volume of a gas at a different temperature or pressure.
What are some applications of the combined gas law?
The combined gas law has a wide range of applications in chemistry, including analyzing gas reactions, designing experiments, and understanding the behavior of gases in industrial processes.