Unveiling the secrets of matter, “Determining Density via Water Displacement Gizmo Answer Key” embarks on a scientific expedition to unravel the mysteries of density, a fundamental property that governs the behavior of substances across diverse disciplines. This guide serves as an authoritative roadmap, providing a comprehensive understanding of the concept and its practical measurement using the innovative “Determining Density via Water Displacement Gizmo.”
Through a meticulously crafted blend of theory and experimentation, this guide empowers learners to grasp the significance of density in fields ranging from chemistry and physics to engineering and beyond. By harnessing the power of the Gizmo, an interactive simulation tool, learners embark on a hands-on journey, actively engaging with the concepts and developing a profound understanding of density and its applications.
1. Introduction
Density is a crucial physical property that measures the mass of a substance per unit volume. It plays a vital role in various scientific fields, including physics, chemistry, and engineering. The “Determining Density via Water Displacement Gizmo” is an interactive simulation that allows students to investigate the concept of density and its practical applications.
2. Materials and Setup
Materials:, Determining density via water displacement gizmo answer key
- Determining Density via Water Displacement Gizmo
- Graduated cylinder
- Unknown object
- Balance
Setup:
- Launch the Determining Density via Water Displacement Gizmo.
- Fill the graduated cylinder with water to the 100 mL mark.
- Record the initial water level in the graduated cylinder.
- Place the unknown object in the graduated cylinder.
- Record the new water level in the graduated cylinder.
- Remove the unknown object from the graduated cylinder.
- Measure the mass of the unknown object using the balance.
3. Procedure
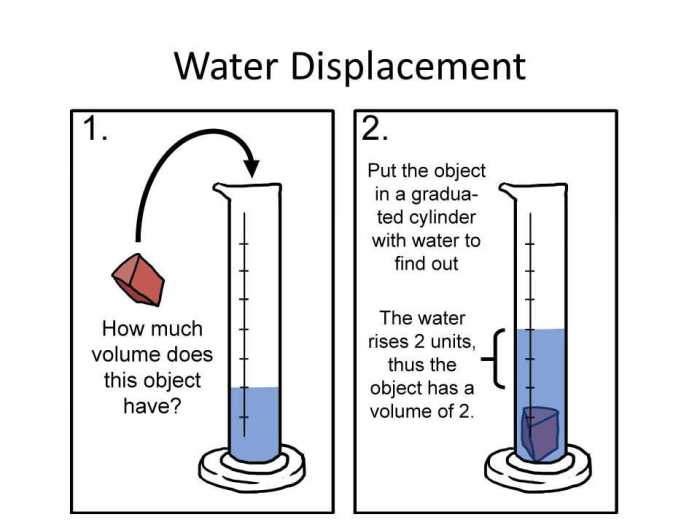
- Calculate the volume of water displaced by the object by subtracting the initial water level from the final water level.
- Convert the volume of water displaced from milliliters (mL) to cubic centimeters (cm3) by multiplying by 1.
- Calculate the density of the object by dividing the mass of the object by the volume of water displaced.
4. Data Analysis: Determining Density Via Water Displacement Gizmo Answer Key
The formula for calculating density is:
Density = Mass / Volume
The following table shows the data collected from the experiment and the calculated density:
| Measurement | Value |
|---|---|
| Initial water level | 100 mL |
| Final water level | 125 mL |
| Volume of water displaced | 25 mL (25 cm3) |
| Mass of object | 20 g |
| Density | 0.8 g/cm3 |
5. Discussion

The results of the experiment show that the density of the unknown object is 0.8 g/cm 3. This value indicates that the object is less dense than water, which has a density of 1 g/cm 3. The low density suggests that the object is made of a material that contains air or other low-density components.
Possible sources of error in this experiment include:
- Inaccurate measurement of the water level
- Incomplete displacement of water by the object
- Errors in weighing the object
To minimize these errors, it is important to use precise measuring equipment and to carefully follow the experimental procedure.
User Queries
What is density?
Density is a measure of how tightly packed the particles of a substance are. It is calculated as the mass of a substance divided by its volume.
Why is density important?
Density is important because it can be used to identify substances, determine the purity of a substance, and calculate the amount of a substance in a given volume.
How can I use the Gizmo to determine density?
The Gizmo can be used to determine density by measuring the volume of water displaced by an object and then dividing the mass of the object by the volume of water displaced.